booth No:1301
GoldenWing (Suzhou) Medical Technology Co., Ltd.

| Company Name | GoldenWing (Suzhou) Medical Technology Co., Ltd. |
|---|---|
| Department | International |
| Address | No. 200 Xingpu Road, Suzhou Industrial Park, Suzhou City, Jiangsu Province, China |
| Tel | 0512-62600881 |
| URL | https://www.goldenwing-glp.com/ |
| Overview | As a core member of Medphoenix Medical Group, GoldenWing Medical was founded in 2018 by renowned Chinese doctors with a mission to become a global leading one-stop service provider for innovative medical devices, offering specialised services including medical research, pre-clinical large animal testing, clinical trials and global registration. We have gathered more than 200 medical doctors with PhD and/or MSc degrees from hospitals in the country and abroad. Our laboratories cover an area of 18,000 square metres and are equipped with a complete set of high-end medical equipment, including renowned brands such as Philips, Siemens, GE, Solin, Mindray, etc. The certification system includes OECD GLP, FDA GLP and CNAS-CL06. We've served over 1,000 domestic and international customers and over 100 partner hospitals. In the field of preclinical studies, we have been ranked No.1 in China. |
| Category | Metal Processing, Metal Parts/Materials Plastic Processing, Plastic Parts/Materials Finished Parts Resin, Ceramic, Rubber Pump, Valve, Pneumatic Component Tubes, Pipes, Needles, Pins, Tubing Materials Filters, Films, Non-woven Fabrics, Tapes Adhesives, Welding and Bonding Technique Surface Treatment, Coating Sterilizing, Sterile Clothing, Aseptic Room Biomaterial Combination Product Measurement, Inspection, Examination, Analytical Instrument Electronic Parts, Electronic Devices Sensors, Software, Application Automation, Assembling, Robot Technology Certification Authority, Consulting Test Kits/Parts Nursing Care/Rehabilitation Robot& Instruments/Parts Rehabilitation Apparatus, Massage Apparatus, Instrument/Parts Ultrasonic waves, VHF therapy equipment, Thermotherapy |
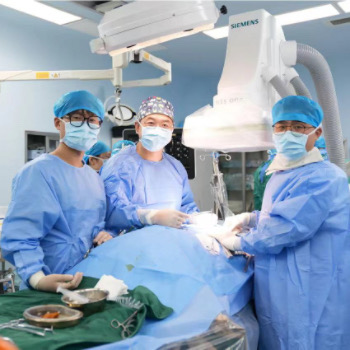
| Exhibitor Description | GoldenWing (Suzhou) Medical Technology Co., Ltd., a leading preclinical medical device testing center established in 2018, will showcase its cutting-edge capabilities in large-animal experimentation at Medtec 2025. Based in Suzhou Industrial Park, our 4,700㎡ state-of-the-art facility specializes in evaluating high-risk innovations such as brain-computer interfaces, implantable artificial hearts, and transcatheter heart valves.
Led by Dr. Wei Xufeng, a National Science and Technology Progress Award laureate and renowned cardiothoracic surgeon, our team of 100+ experts achieves a 90% success rate in complex procedures, including transcatheter valve implantation. We highlight our CNAS/OECD GLP-certified labs equipped with advanced imaging systems (Philips 128-slice CT, Siemens Artis zee), hybrid operating theaters, and an ICU suite valued at ¥50 million. Recognized as a Top 50 Most Valuable Medtech Company (2024) and a national innovation alliance platform, we deliver end-to-end solutions—from animal trials to regulatory submissions—ensuring seamless translation of medical breakthroughs. At Medtec 2025, explore our live demonstrations of preclinical workflows, discuss customized testing protocols, and discover how we empower your devices to meet global regulatory standards. Join us to advance healthcare innovation through excellence in translational research! |
Preclinical animal testing of innovative medical devices
その他